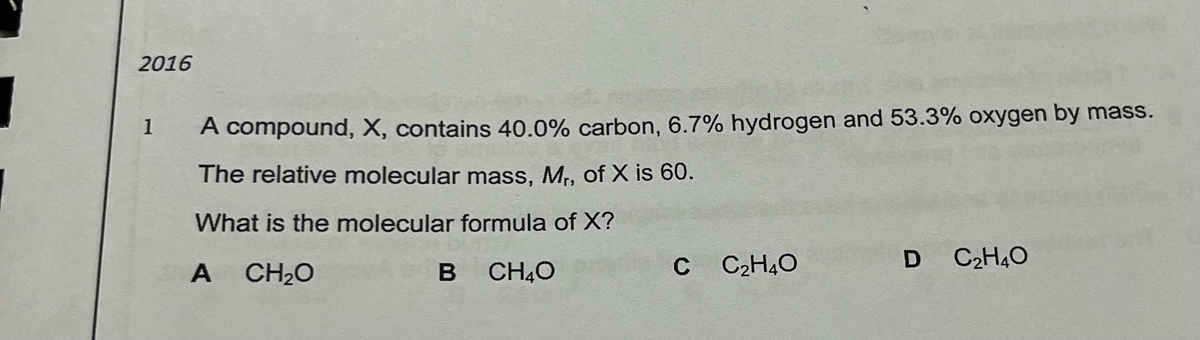An organic compound containing C , H and O gave the following percentage composition: C = 40.68% , H = 5.08% . The vapour density of the compound is 59 . Calculate the molecular formula of the compound.

An organic compound contain carbon =40 percent, hydrogen =6.67 percent and remain oxygen. If the molecular - Brainly.in
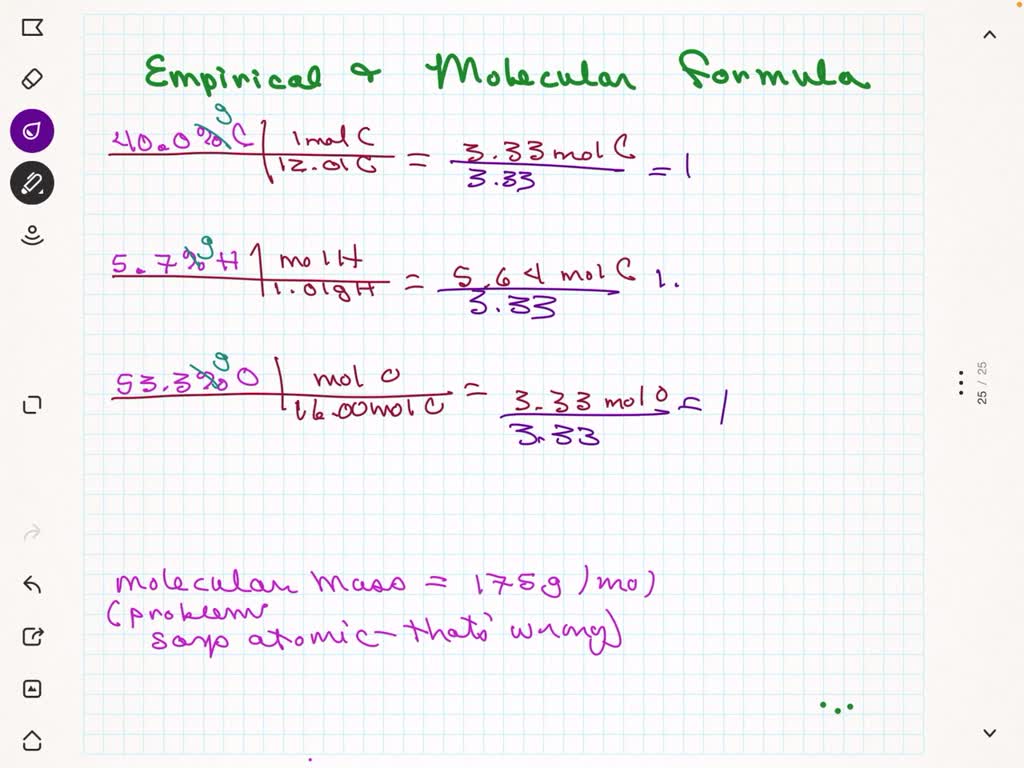
SOLVED: A compound containing 40.0% carbon, 5.7% hydrogen, and 53.3% oxygen has an atomic mass of 175 g/mol. What is the molecular formula?

CH135 Molecular Formula.docx - CH135 Molecular Formula Question 1 An unknown compound is found to contain 40.0% carbon 6.7% hydrogen and 53.3% oxygen | Course Hero
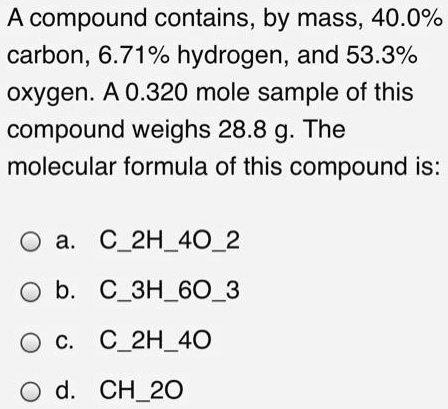
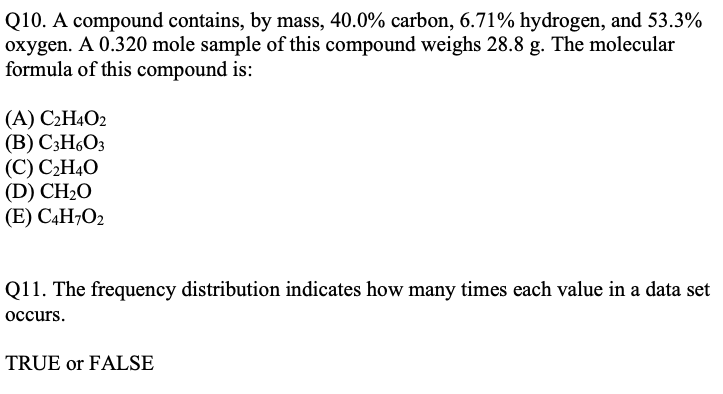
SOLVED: compound contains, by mass, 40.0% carbon, 6.71% hydrogen, and 53.3% oxygen: A 0.320 mole sample of this compound weighs 28.8 g The molecular formula of this compound is: a C2H402 b

3.37b | Determine the empirical formula for a compound with 40.0% carbon, 6.7% hydrogen, and 53.3% - YouTube

What is the empirical formula for a compound containing 38.8% carbon, 16.2% hydrogen and 45.1% nitrogen?
A compound has a molar mass of approximately 180 g/mol and a percent composition of 40.00% Carbon, 6.72% Hydrogen, and 53.29% Oxygen. What is the molecular formula of the compound? - Quora
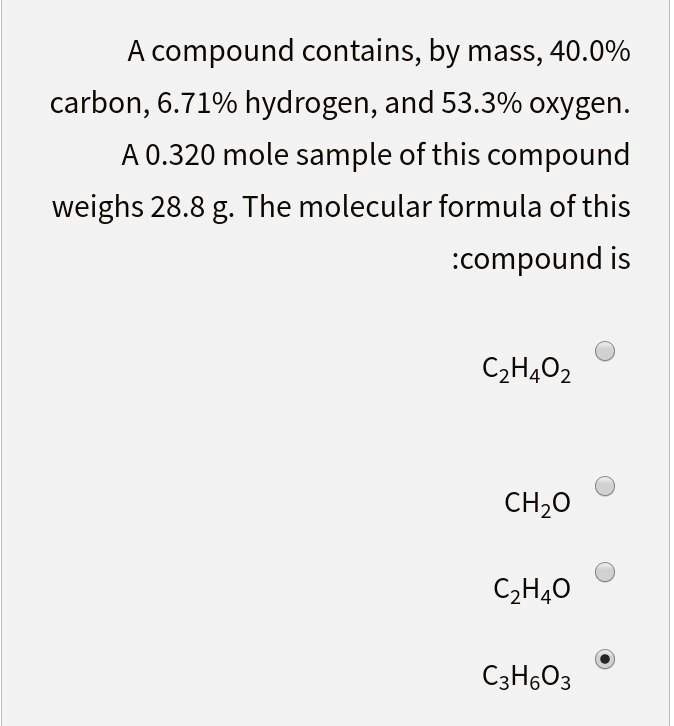
SOLVED: A compound contains, by mass, 40.0% carbon, 6.71% hydrogen, and 53.3% oxygen A 0.320 mole sample of this compound weighs 28.8 g The molecular formula of this :compound is C2H40z CHzO CzHao CzHoO3

Determine the empirical formula of a compound that contains 40.0% C, 6.7% H, and 53.3% O by mass. - YouTube

3 A compound containing Carbon, Hydrogen and Oxygen gave the following analytical data: Carbon=40 0% , Hydrogen =6 67% - Chemistry - Some Basic Concepts of Chemistry - 12771131 | Meritnation.com

SOLVED: A compound contains, by mass, 40.0% carbon, 6.71% hydrogen, and 53.3% oxygen A 0.320 mole sample of this compound weighs 28.8 g The molecular formula of this :compound is C2H40z CHzO CzHao CzHoO3
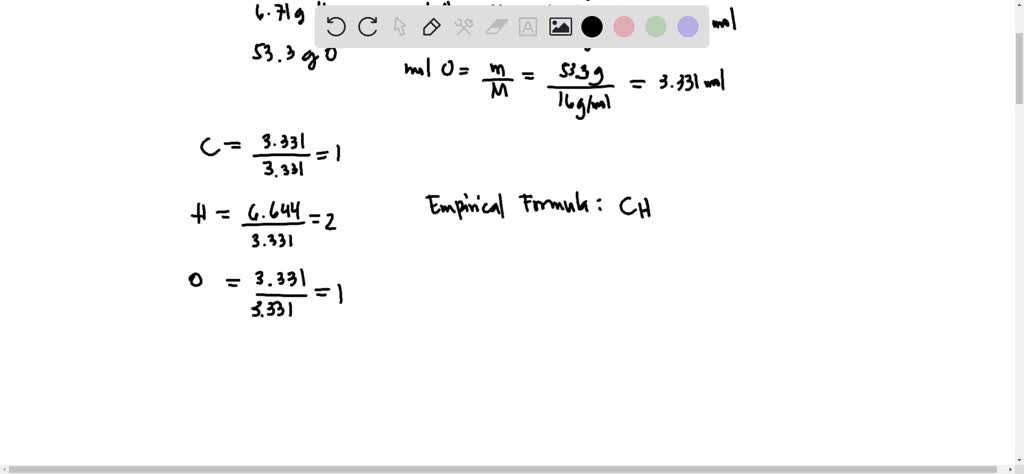
SOLVED: compound contains, by mass, 40.0% carbon, 6.71% hydrogen, and 53.3% oxygen: A 0.320 mole sample of this compound weighs 28.8 g The molecular formula of this compound is: a C2H402 b







![Punjabi] A compound containing carbon, hydrogen and oxygen gave the f Punjabi] A compound containing carbon, hydrogen and oxygen gave the f](https://d10lpgp6xz60nq.cloudfront.net/physics_images/OMG_CHE_XI_C01_E01_083_S01.png)