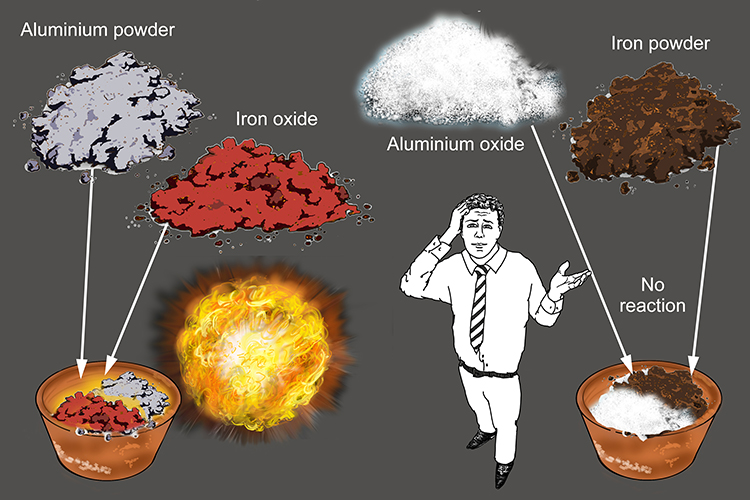
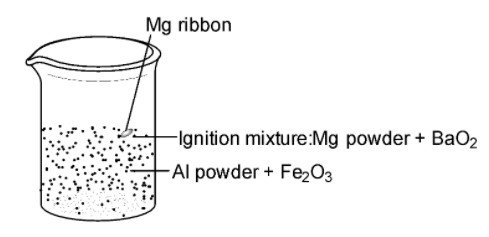
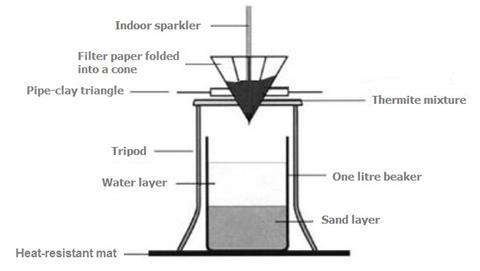
SOLVED: When a mixture of aluminium powder and iron (III) oxide is ignited, it produces molten iron and aluminium oxide. In an experiment, 5.4 g of aluminium was mixed with 18.5 g

SOLVED: Fe2O3(s) + 2Al(s) –> Al2O3(s) + 2Fe(s) Mass of iron oxide powder = 2.63g Mass of aluminium powder = 0.83g Using the given masses of reactants above, which reactant is the

SOLVED: The thermite reaction has solid aluminum powder (28.871 J(mol-K ) reacting with iron(Ill) oxide (87.793 J(mol-K to make aluminum oxide (49.56 J(mol-K ) and iron (25.305 J(mol:K) The reaction is So

when a mixture of aluminium powder and iron (III) oxide is ignited, it produces molten iron and - YouTube











:max_bytes(150000):strip_icc()/GettyImages-578813868-5a787515a9d4f90036eff588.jpg)