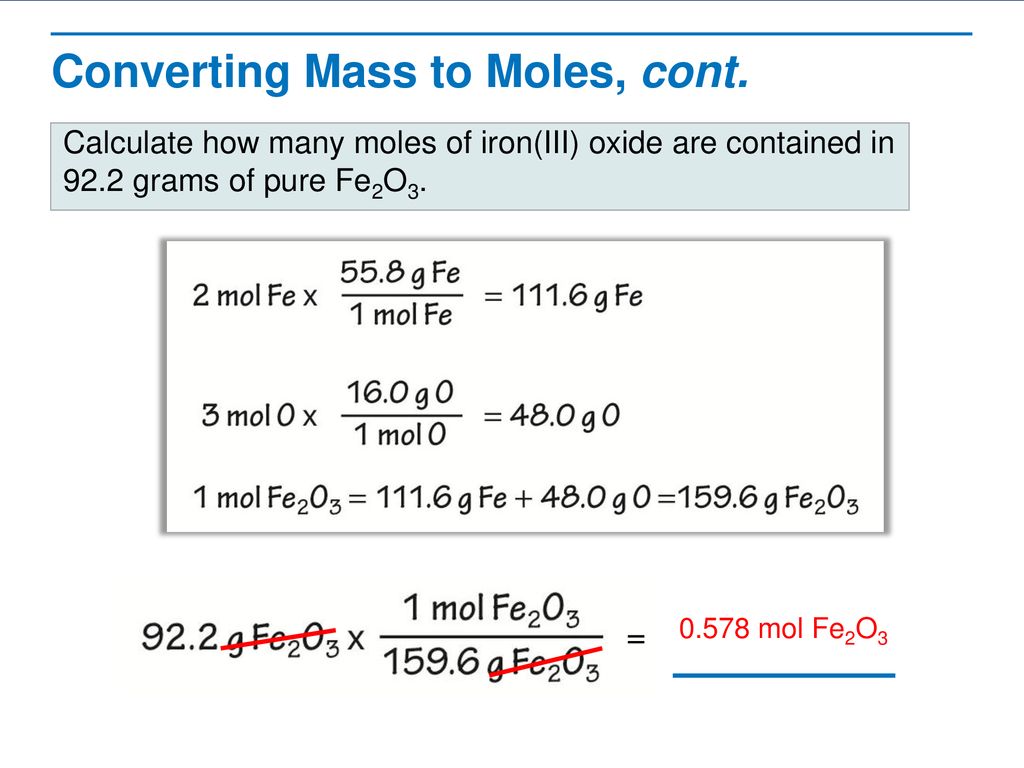
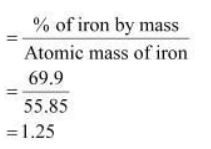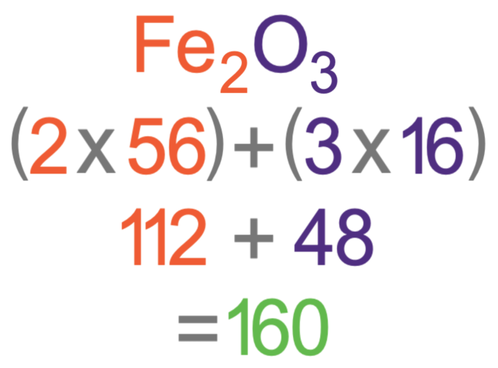Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.

Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively. Given that molecular mass is 159.69g.
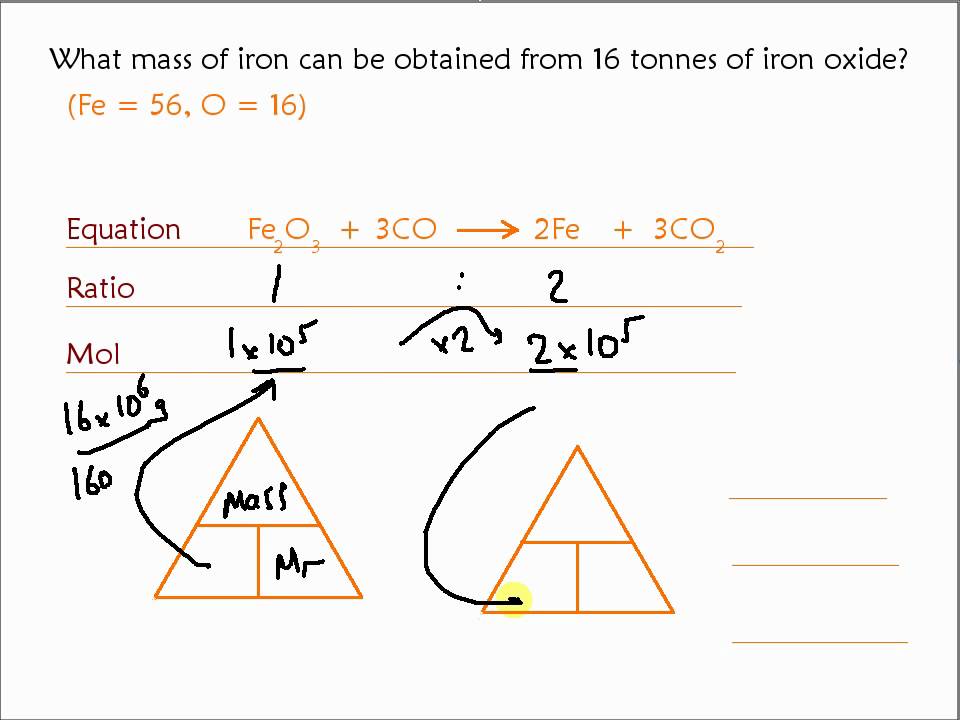
Iron is produced by the reduction of iron (III) oxide using carbon monoxide. Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO2(g). How much Fe is produced from 1 kg of Fe2O3? - Quora

Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively.

Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively.