Chem Lab 100ML Barium standard solution (Plasma HIQU)(1.44 g BaCO3 / l 2% HNO3) Products | Fisher Scientific

Process Intensification in Nitric Acid Plants by Catalytic Oxidation of Nitric Oxide | Industrial & Engineering Chemistry Research
Cho dung dịch Ba(HCO3)2 lần lượt vào các dung dịch: CuSO4, NaOH, NaHSO4, K2CO3, Ca(OH)2, H2SO4, HNO3
Electrodeposition of BaCO3 coatings on stainless steel substrates: Oriented growth in the presence of complexing agents

SOLVED: 1. BaCO3+HNO3 -> Ba(NO3)2+ CO2+H2O a. oxidation-reduction b. precipitationc. acid-base neutralization 2. Al(NO3)3+Na2CO3 -> Al2(CO3)3+NaNO3a. oxidation-reduction b. precipitationc. acid-base neutralization



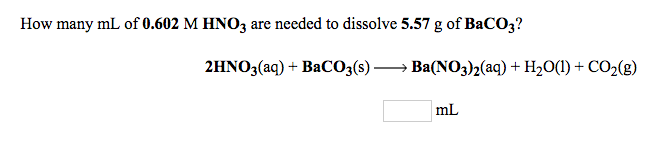





![ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/75663599-1659635277.7061477.jpeg)