Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
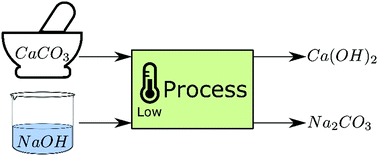
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate - Energy & Environmental Science (RSC Publishing)

CO2 capture by aqueous Na2CO3 integrated with high-quality CaCO3 formation and pure CO2 release at room conditions - ScienceDirect

Question Video: Identifying the Chemical Equation- with State Symbols- That Corresponds to a Chemical Statement | Nagwa

Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram

Microbially/CO2-derived CaCO3 cement strengthens calcareous sands and its cementation mechanism | SpringerLink
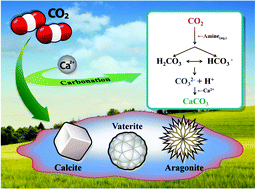
CO2 mineralization into different polymorphs of CaCO3 using an aqueous-CO2 system - RSC Advances (RSC Publishing)
If 220 grams of calcium oxide (CaO) reacts with 50L of carbon dioxide (CO2), what mass of calcium carbonate (CaCO3) is produced? - Quora
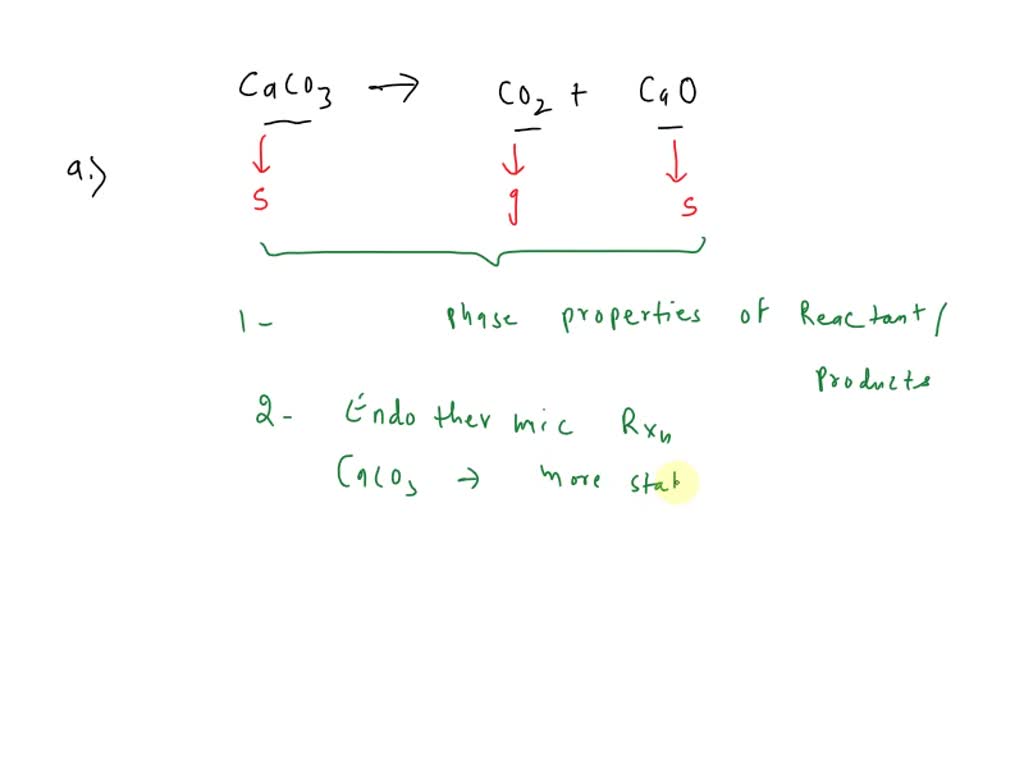
SOLVED: Calcium carbonate is a white mineral. A chemist burns calcium carbonate, CaCO3, to produce carbon dioxide, CO2, and a white powder, CaO. The following chemical equation shows the reaction. CaCO3 ———>

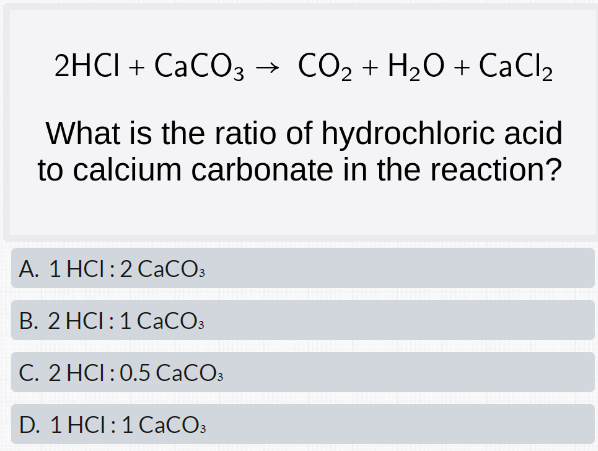
How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O | How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O Hey there! Are you struggling with balancing







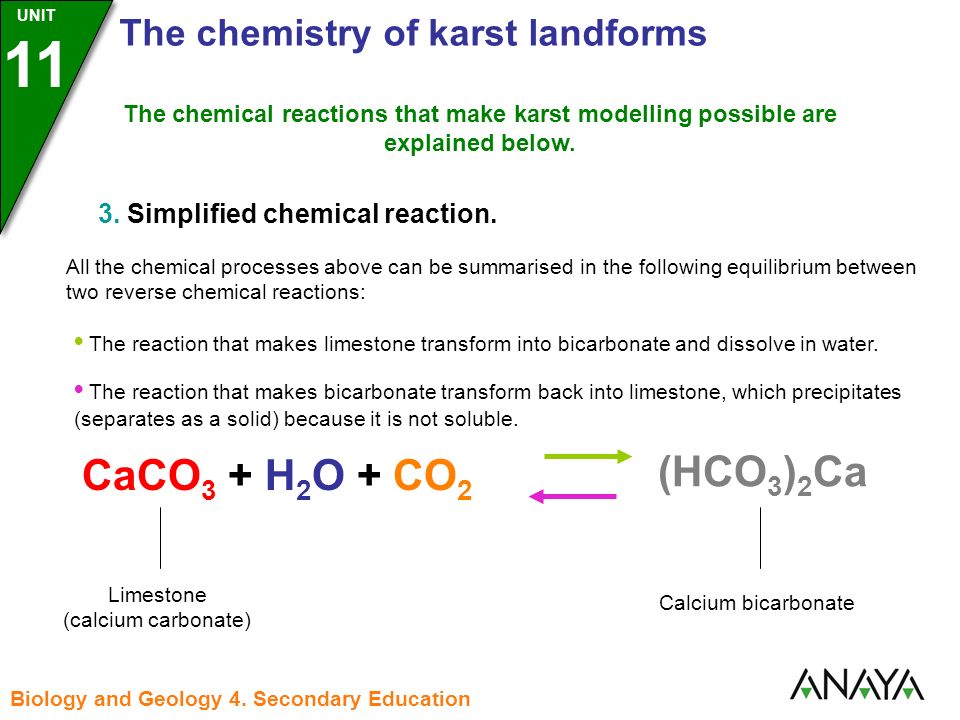

![PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/70ca22dce93c5e0c4e267a02ccc4e41951e44132/2-Figure1-1.png)