.bmp)
The C-C length of Diamond is more than that of graphite, then also it is harder than Graphite instead of - Science - Carbon and its Compounds - 4350177 | Meritnation.com
New Carbon Allotropes with Helical Chains of Complementary Chirality Connected by Ethene-type π-Conjugation | Scientific Reports
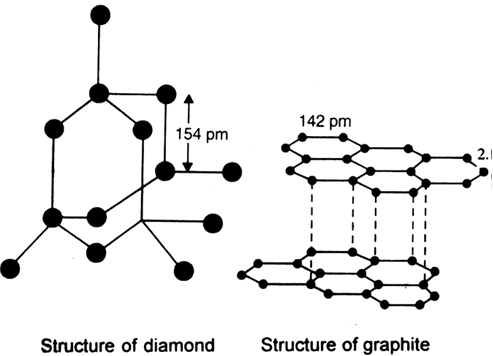
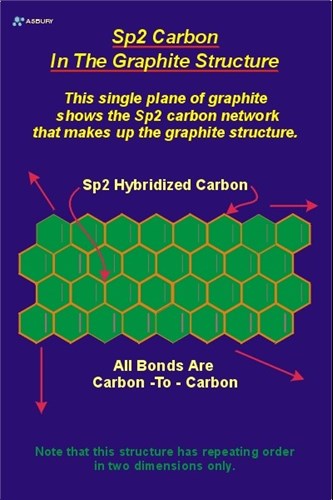
What are allotropes? Sketch the structure of two allotropes of carbon family namely diamond and graphite. What is the impact of structure on physical properties of two allotropes? from Chemistry The p-Block
The $\\text{ C}-\\text{C }$ bond length is maximum in:A) GraphiteB) $\\text{ }{{\\text{C}}_{\\text{70}}}\\text{ }$C) DiamondD) $\\text{ }{{\\text{C}}_{\\text{60}}}\\text{ }$
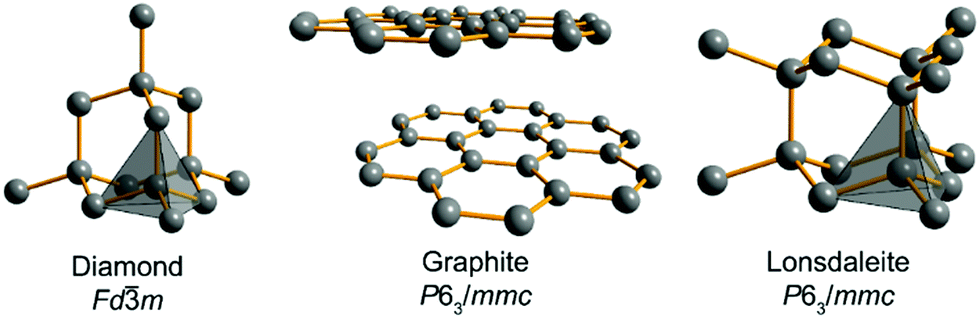
Relative stability of diamond and graphite as seen through bonds and hybridizations - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C8CP07592A

Schematic diagram for the structure of the B-H bc complex in diamond.... | Download Scientific Diagram

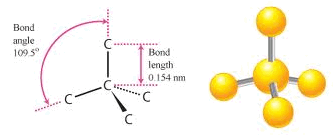
Crystallographic structure of diamond with tetrahedral bond angles of... | Download Scientific Diagram

1: The unit cell of diamond, showing the bond lengths and tetrahedral... | Download Scientific Diagram

If C-C bond length in diamond is `1.5Å`, then which be the edge length of the cubic close packed - YouTube