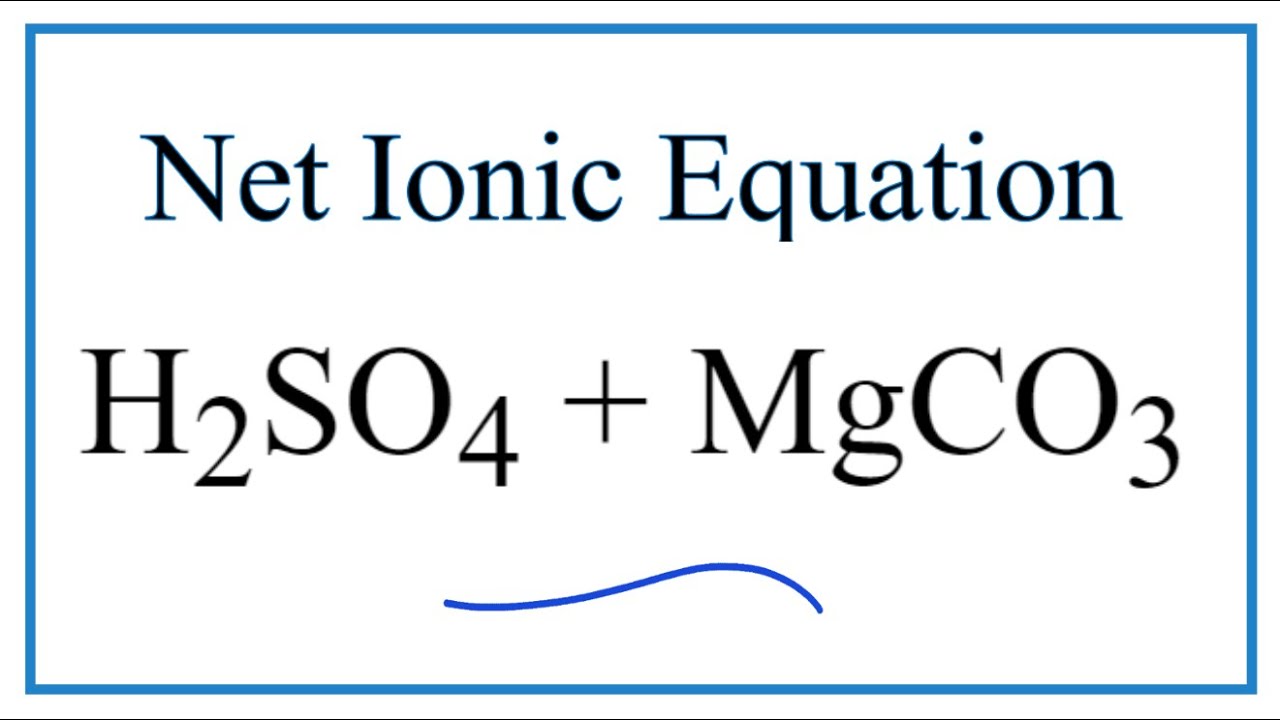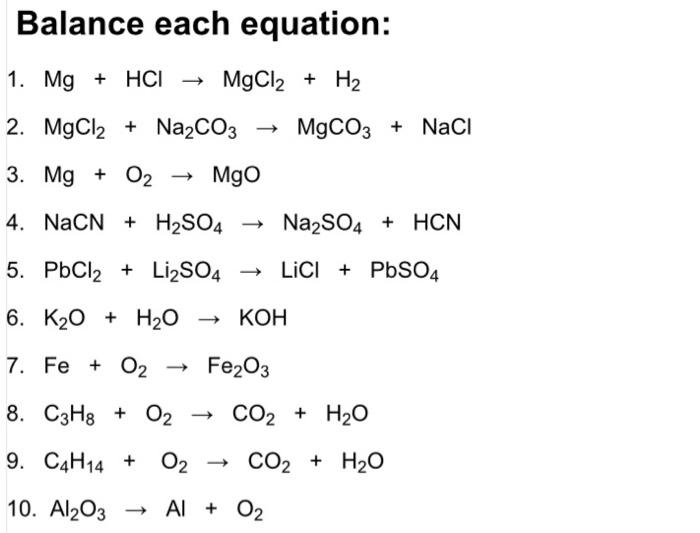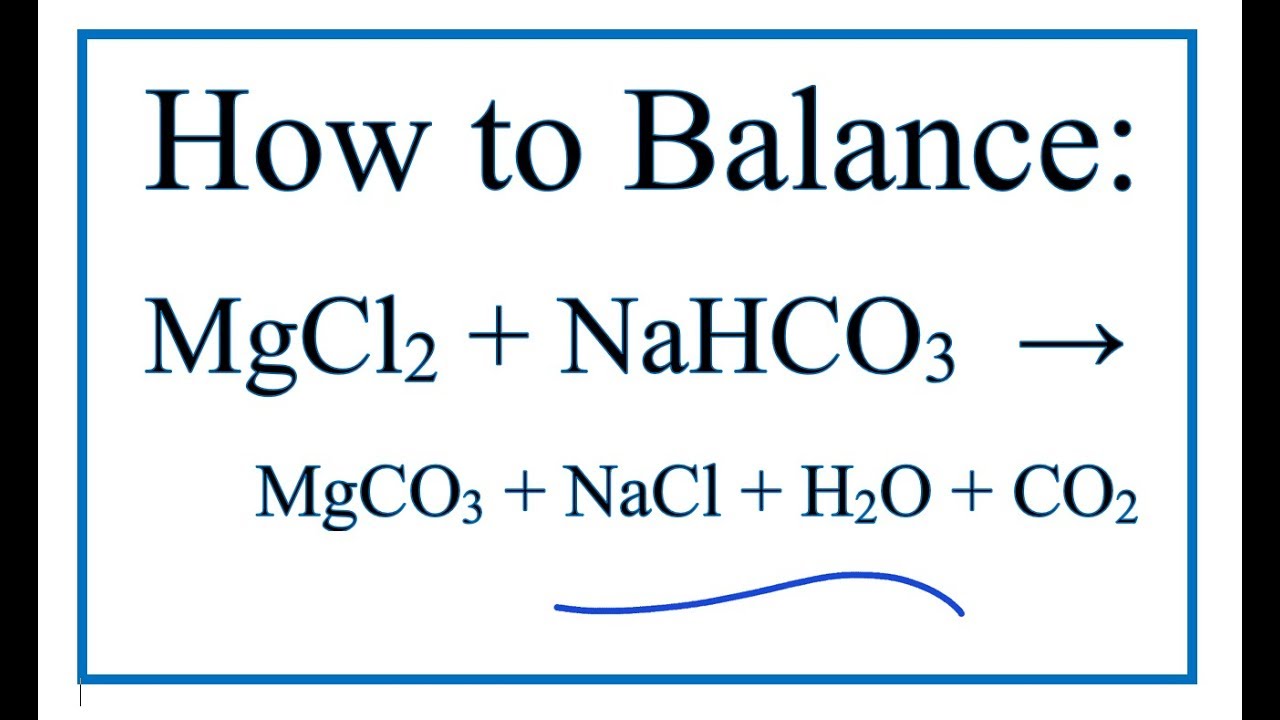
How to Balance MgCl2 + NaHCO3 = MgCO3 + NaCl + H2O + CO2 | Magnesium chloride + Sodium bicarbonate - YouTube
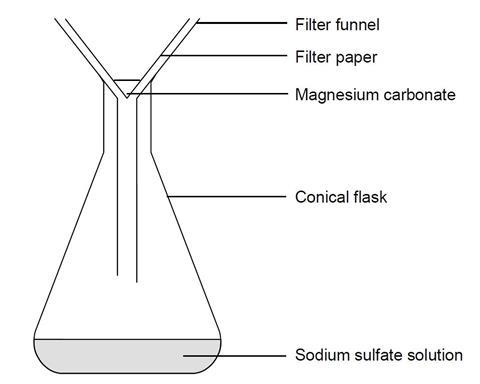
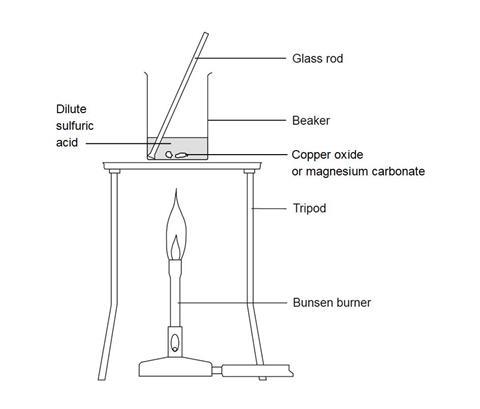
Making magnesium carbonate: the formation of an insoluble salt in water | Experiment | RSC Education

Chemical composition of the magnesium carbonate (MgCO 3 ), magnesium... | Download Scientific Diagram
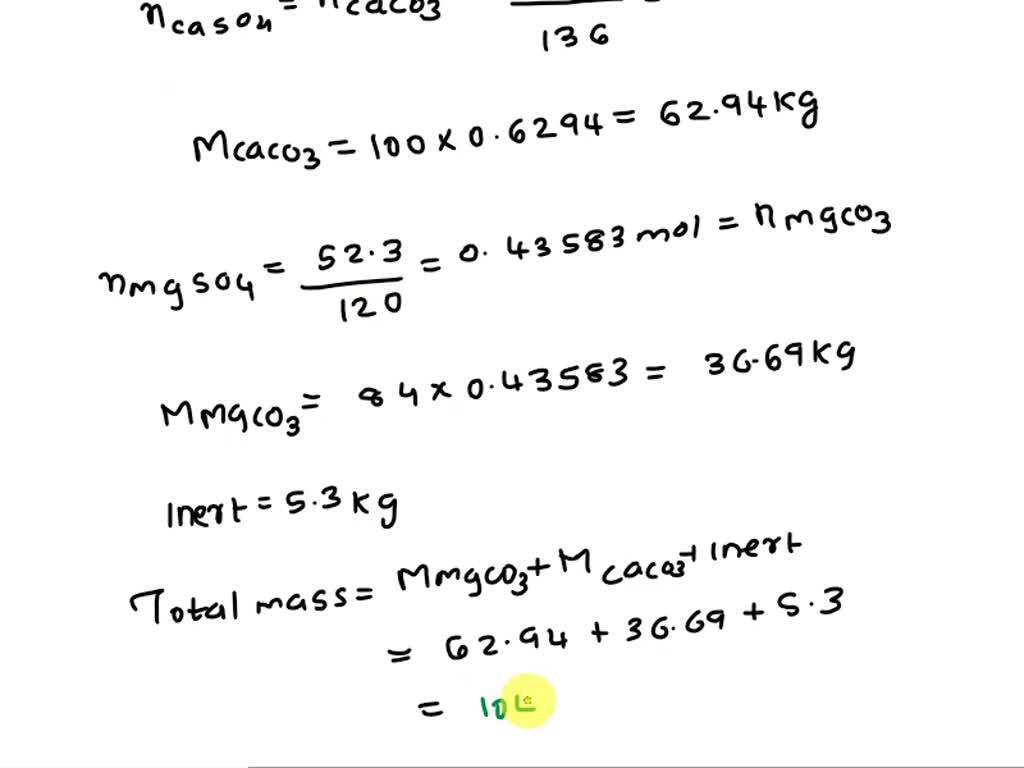
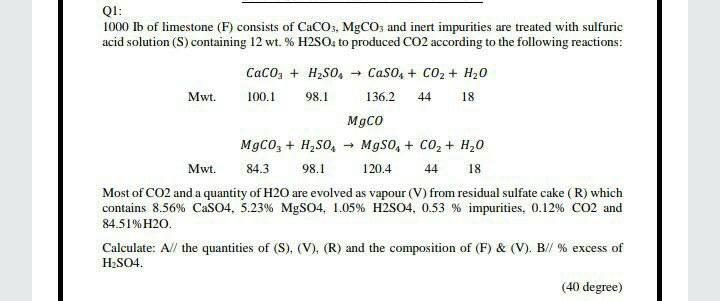
SOLVED: Pure CO2 may be prepared by treating limestone with sulfuric acid. The limestone used in the process contains CaCO3, MgCO3 and inert. The acid used contains 12% H2SO4 by weight The
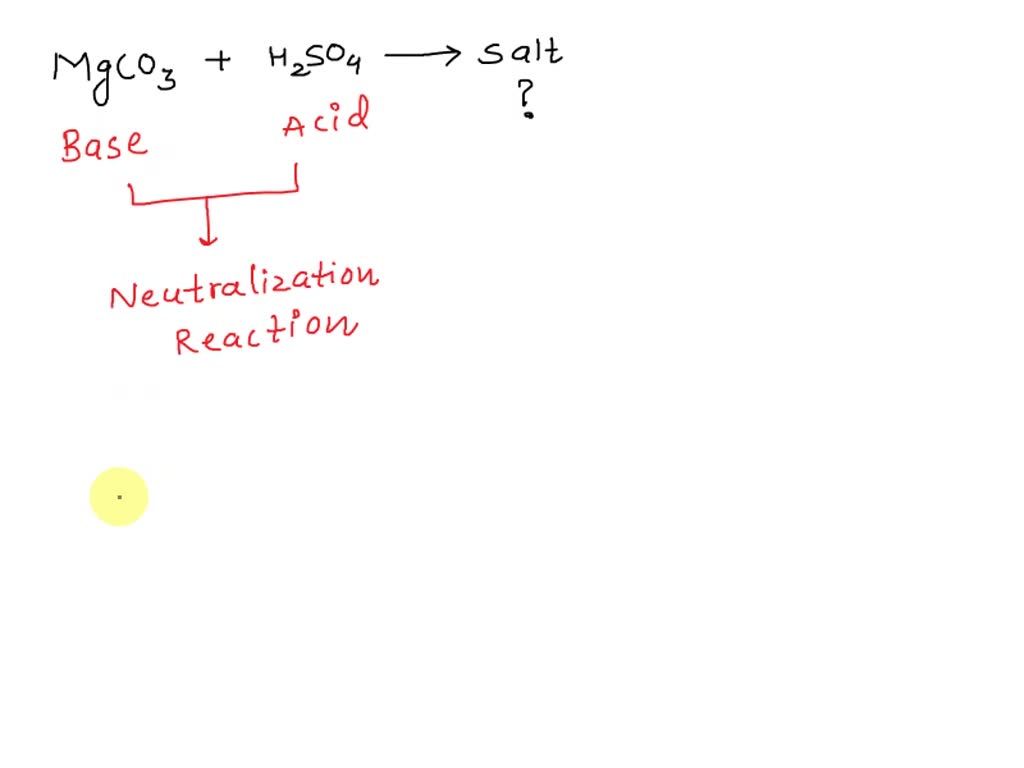
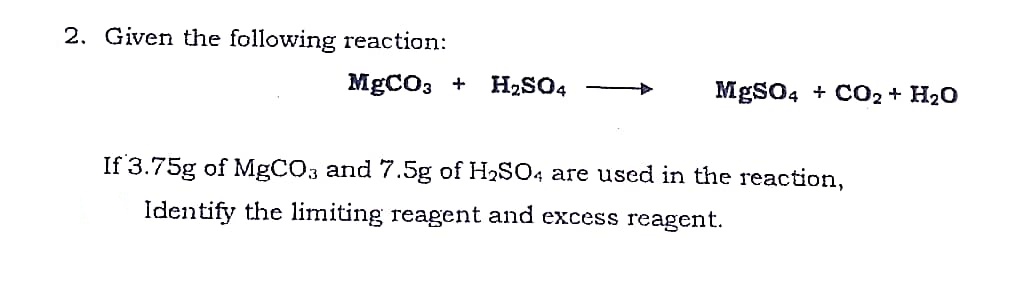
SOLVED: Which salt is made when magnesium carbonate MgCO3 reacts with sulfuric acid H2SO4? (2 Points) Mognesiurn Chloride MgC12 Magnasium Nittate MalNO3) Magnesiumn Sullate Mg S04 Magnesiumn hypochlatite Mg (CIOJ2

How to balance MgCO3+H2SO4=MgSO4+CO2+H2O|Reaction balance MgCO3+H2SO4=MgSO4+CO2+H2O| MgCO3+H2SO4= - YouTube
What weight of sulphuric acid will be required to completely dissolve 3g of magnesium carbonate? - Sarthaks eConnect | Largest Online Education Community