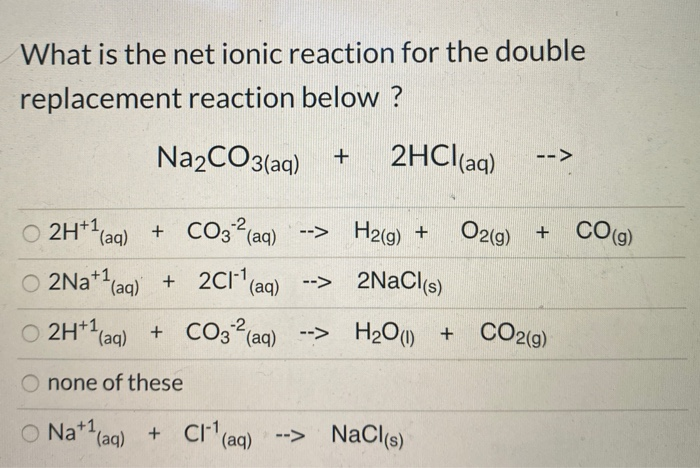
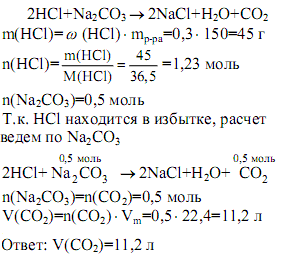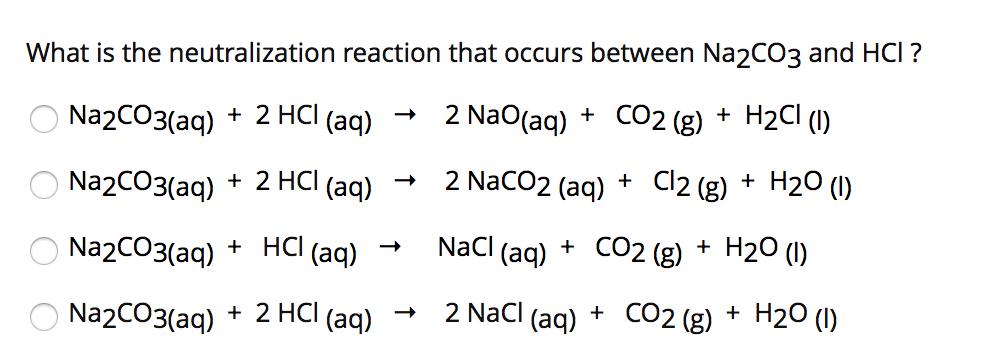SOLVED: Consider the reaction between Na2CO3 and HCl: 2HCl(aq) + Na2CO3(aq) ⟶⟶ H2O(l) + CO2(g) + 2NaCl(aq) When 16.3 g of HCl reacts with 17.2 g of Na2CO3, 4.33 g of CO2

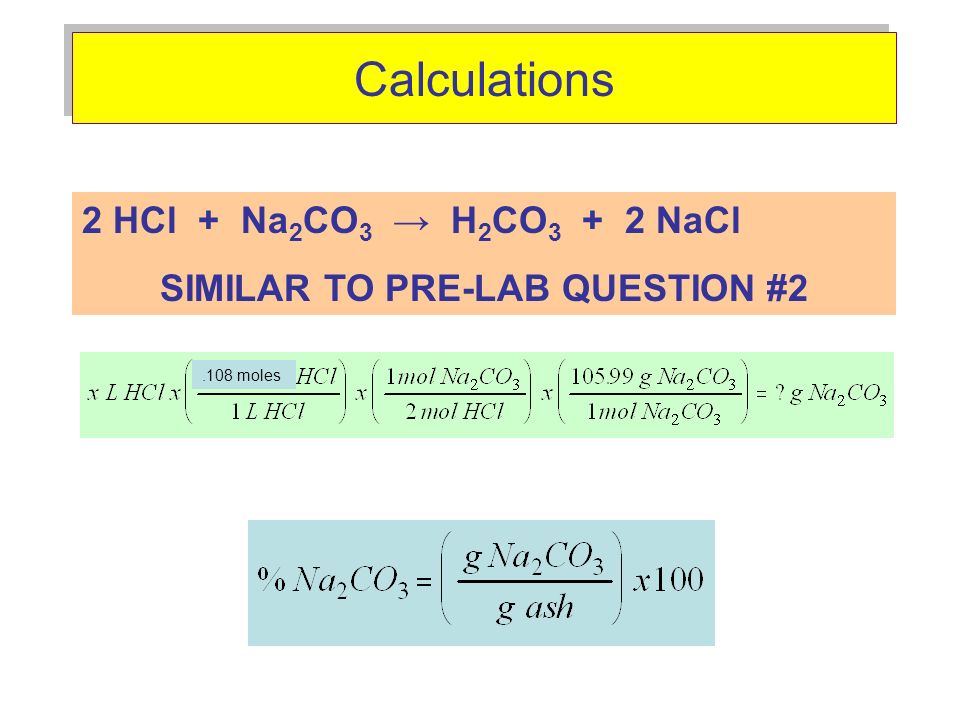
Solved! How many liters of 0.53 M HCl is required to neutralize 0.78 g of sodium carbonate (Na2CO3)? (MM of Na2CO3 = 105. 99 g/mol) 𝟐𝑯𝑪𝒍 + 𝑵𝒂𝟐𝑪𝑶𝟑 → 𝟐𝑵𝒂𝑪𝒍 +

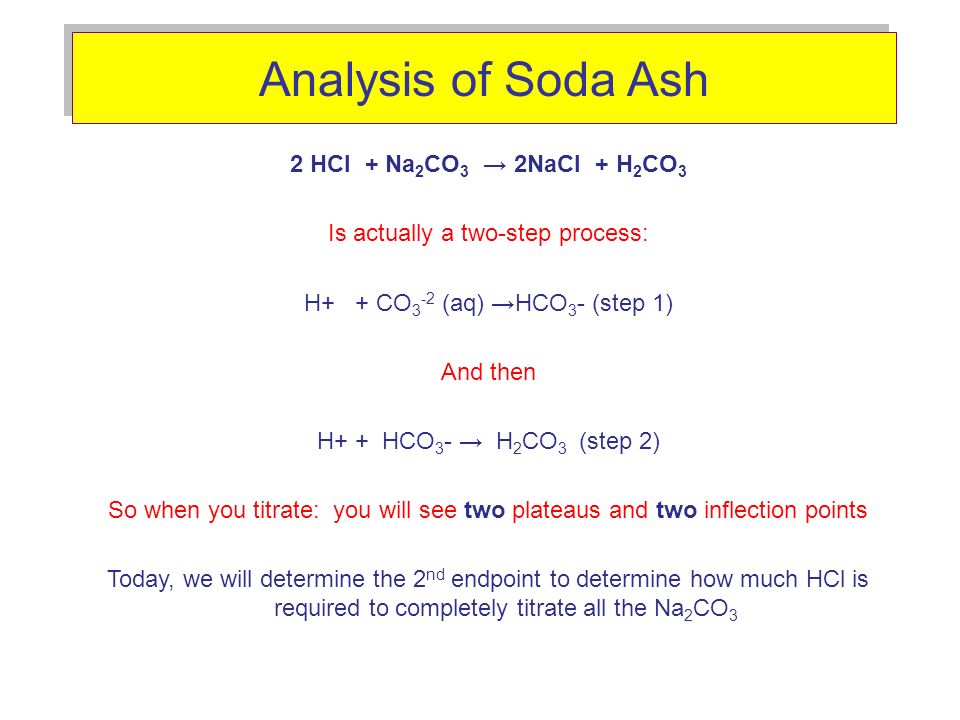
We can measure the concentration of HCl solution by its reaction with pure sodium carbonate. 2 H+ + Na2CO3 ? 2 Na+ + H2O + CO2 Complete reaction with 0.9639 0.0005 g of Na2CO3 required 28.20 0. | Homework.Study.com

What are the tricks to learn reaction of sodium carbonate with hydrochloric acid? – The Unconditional Guru

Question Video: Identifying the Observations of the Reaction between Hydrochloric Acid and Sodium Carbonate | Nagwa

SOLVED: Hydrochloric acid (HCl) reacts with sodium carbonate (Na2CO3), forming sodium chloride (NaCl), water (H2O), and carbon dioxide (CO2). This equation is balanced as written: 2HCl(aq)+Na2CO3(aq)→2NaCl(aq)+H2O(l)+CO2(g) Part A What volume of 1.75
What will be the mass of sodium chloride formed when 5.3 g of sodium carbonate is dissolved in 250 ml of a half molar HCl solution? - Quora