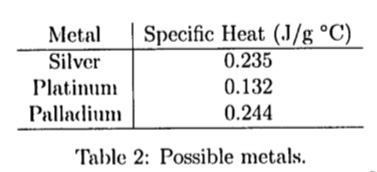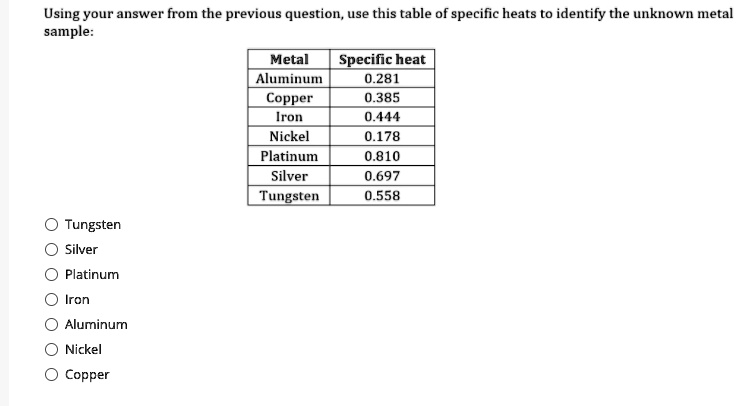
SOLVED: Using your answer from the previous question use this table of specific heats to identify the unknown metal sample: Metal Specific heat Aluminu 0.281 Copper 0.385 Iron 0.444 Nickel 0.178 Platinum
![PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic](https://d3i71xaburhd42.cloudfront.net/5c9e069d85ccf05c4d6c61615da485b98eeea7b6/9-Table1-1.png)
PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic
![PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic](https://d3i71xaburhd42.cloudfront.net/5c9e069d85ccf05c4d6c61615da485b98eeea7b6/22-Table6-1.png)
PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic

SOLVED: Platinum has specific heat capacity of 0.133 Jg: € A The temperature of a piece of pure platinum increases from 26.3*C to 32.8*C as it gains 6.74 J of energy: What

Heat Problems Calorimeter – an instrument used to study the heat of chemical reactions. Calorimetry – the study of the heat of chemical reactions. - ppt download
![PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic](https://d3i71xaburhd42.cloudfront.net/5c9e069d85ccf05c4d6c61615da485b98eeea7b6/13-Table3-1.png)
PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic

Question Video: Finding the Specific Heat Capacity of a Substance given the Change in Its Temperature and Internal Energy | Nagwa
![PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic](https://d3i71xaburhd42.cloudfront.net/5c9e069d85ccf05c4d6c61615da485b98eeea7b6/12-Table2-1.png)
PDF] Critical Analysis of Heat—Capacity Data and Evaluation of Thermodynamic Properties of Ruthenium, Rhodium, Palladium, Iridium, and Platinum from 0 to 300K. A Survey of the Literature Data on Osmium. | Semantic

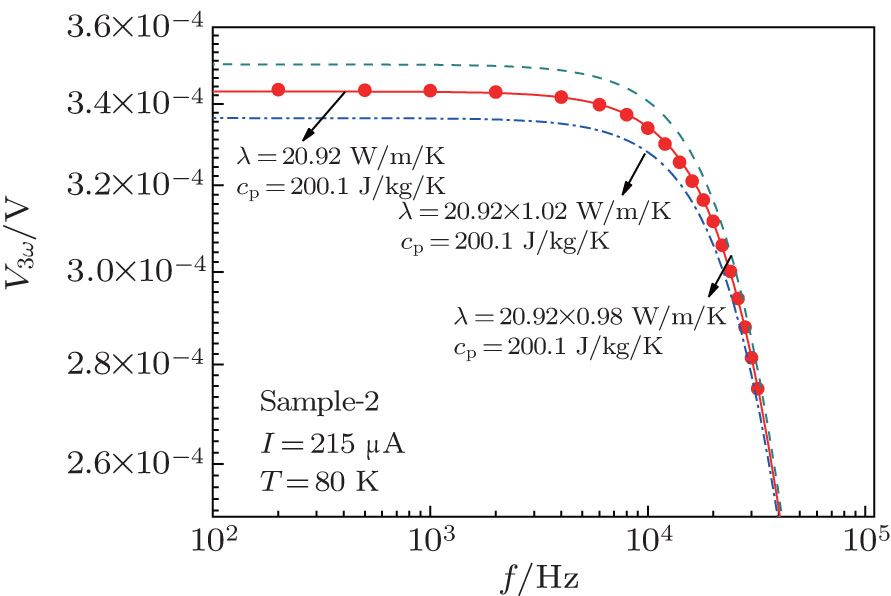

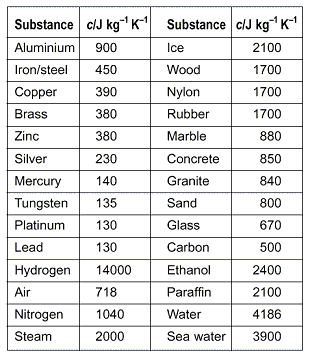



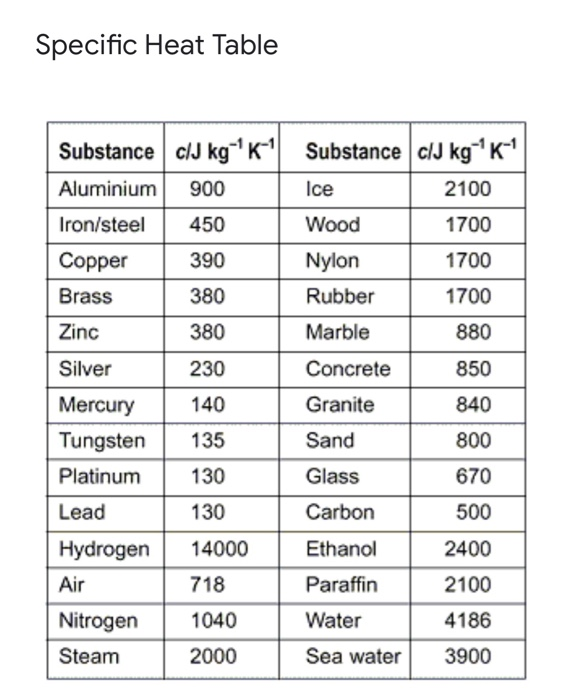
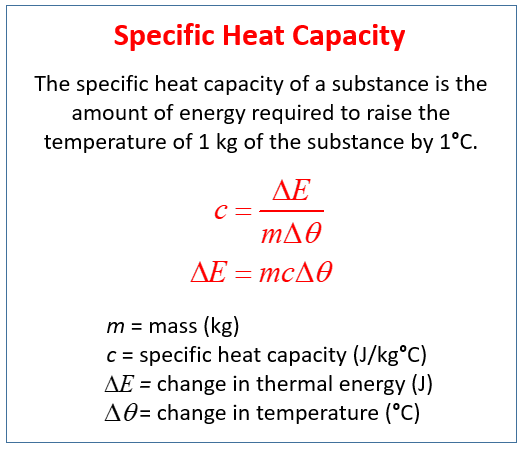

.jpg)

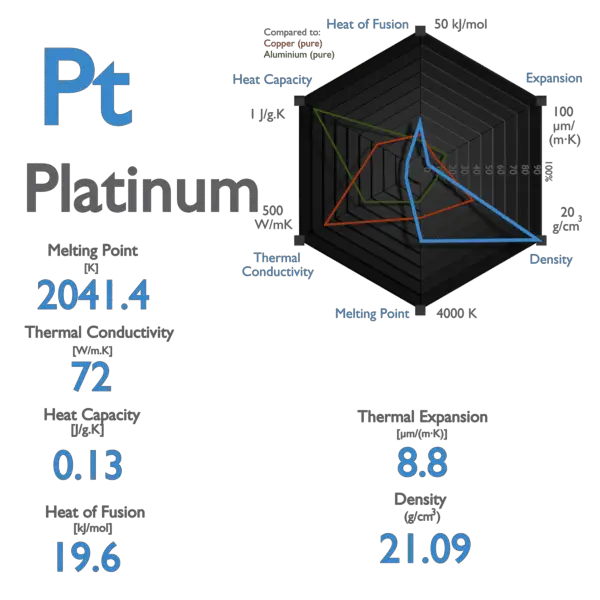
.jpg)